
Research
Confinement Catalysis to Overcome Synthetic Challenges
Our interest is mainly focused on exploring supramolecular host structures, so-called ‘molecular flasks’, for catalysis. The main goal is not only to perform chemistry in these host structures, but to find solutions for current challenges in synthetic organic chemistry that are difficult to address with traditional tools. Currently, the focus is mainly on glycosylation chemistry, terpene cyclizations, and C-H oxidation.
Oligosaccharides
Challenge. In contrast to other biopolymers (oligonucleotides and oligopeptides), complex oligosaccharides cannot be ordered online. The synthesis of complex oligosaccharides is still challenging and very difficult to automate. One challenge involves the formation of two diastereoisomers for each glycosidic linkage, the α- and β-isomers:
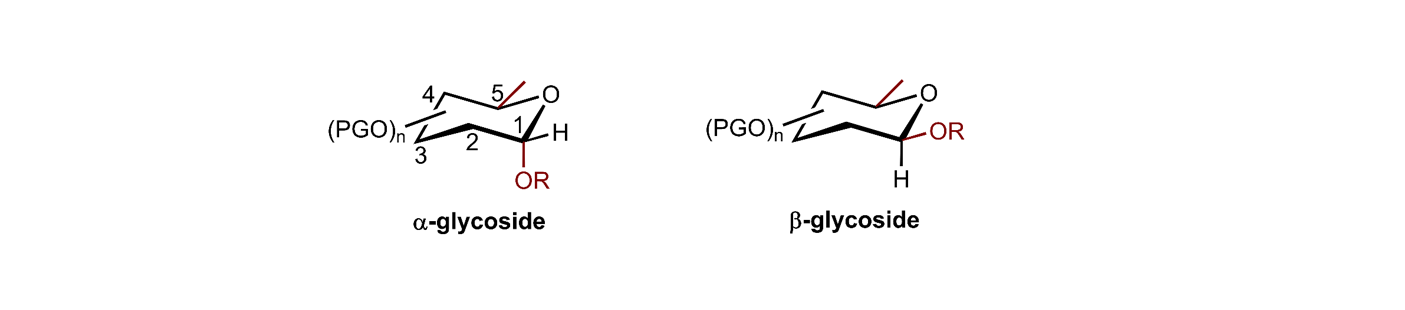 Vision. Creating two catalysts, catalyst-α and catalyst-β, that produce α- and β-glycosidic linkages reliably, independently of the substrates involved and their specific configuration, protecting groups, etc. This would enable a more facile automated synthesis of complex oligosaccharides.
Vision. Creating two catalysts, catalyst-α and catalyst-β, that produce α- and β-glycosidic linkages reliably, independently of the substrates involved and their specific configuration, protecting groups, etc. This would enable a more facile automated synthesis of complex oligosaccharides.
- We recently demonstrated that the glycosylation of a wide range of glycosyl fluorides with diverse O-nucleophiles reliably produces the respective β-glycosides in high selectivity.
J. Am. Chem. Soc. 2023, 145, 4294.
Nat. Chem. 2022, 14, 985.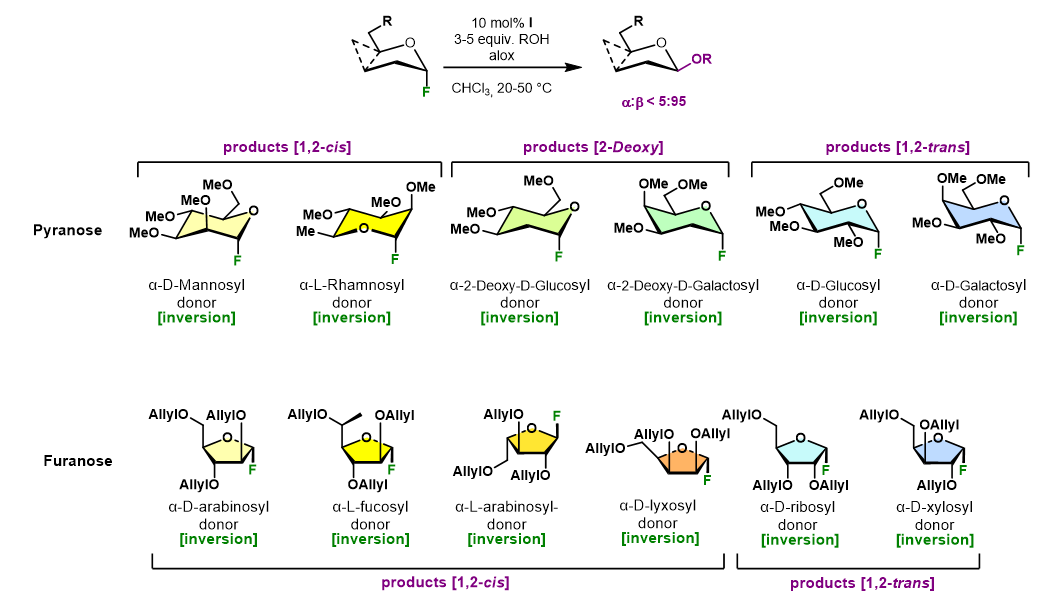
- The same catalyst was able to convert not only diverse pyranosyl electrophiles, but also diverse furanosyl electrophiles. To our knowledge, no alternative catalyst is available that displays such a broad substrate scope.
- Interestingly, high β-selectivity is observed independently of the exact reaction mechanism involved (SN1 vs. SN2).
- However, as the reaction takes place inside a closed molecular flask, there is a strict size limit (disaccharide). We are currently exploring ways of overcoming this size limit while preserving the high selectivity and the broad scope concerning substrate isomers. Thus, for instance, we started to explore larger molecular building blocks:
Angew. Chem. Int. Ed. 2022, 61, e202209885.
JACS Au 2021, 11, 1885.
Terpene Cyclizations
Challenge. Terpenes constitute the largest and most diverse class of natural products, with several tens of thousands of different members. Important current drugs like taxol/paclitaxel (cancer treatment) or artemisinin (an antimalarial drug) have emerged from terpene natural products. However, the synthesis of each terpene skeleton requires the development of a novel synthetic route, making the access to complex terpenes both time- and resource-inefficient.
 Vision. Creating a modular synthesis via predictable cationic cyclizations of simple cyclization precursors.
Vision. Creating a modular synthesis via predictable cationic cyclizations of simple cyclization precursors.
Interestingly, the variety of terpene products in nature is generated from only a handful of relatively simple acyclic precursors: geranyl-pyrophosphate (PP) for monoterpenes (10 C-atoms), farnesyl-PP for sesquiterpenes (15 C-atoms), and geranylgeranyl-PP in case of diterpenes (20 C-atoms). These simple precursors are cyclized via complex cationic reaction cascades, termed tail-to-head terpene (THT) cyclizations, into the whole variety of natural terpene carbon structures. As an example, the biosynthesis of taxol is shown here:
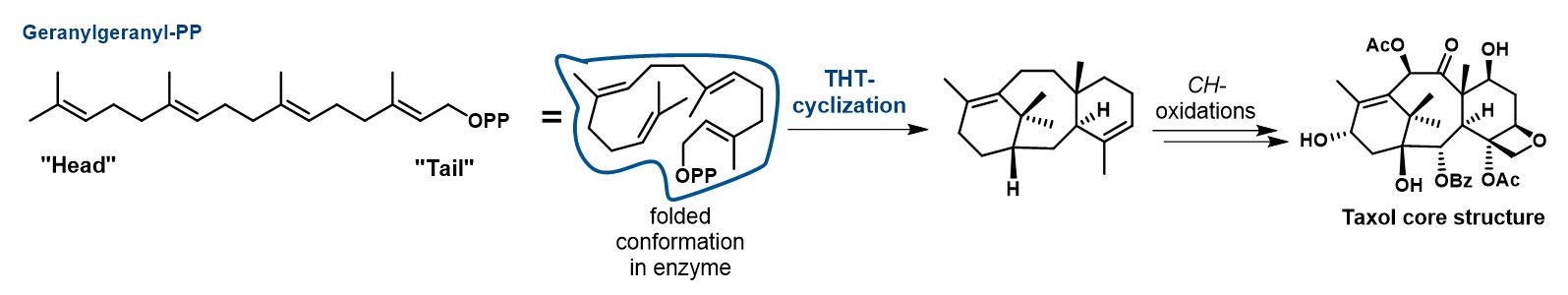
Such THT cyclizations are considered to be among the most complex chemical reactions occurring in nature. Most of the atoms of the acyclic terpene precursor change their hybridization or bonding during the cationic cyclization cascade. Nature has evolved enzymes termed terpene cyclases or synthases to perform this task. However, man-made catalysts for the THT cyclization are lacking. Learning how to design such complex catalysts will not only enable us to mimic natural enzymes, but to enter uncharted territory of terpene chemistry. To influence the conformation of the flexible acyclic terpenes and to stabilize cationic transition states, enzymes surround the substrate more or less completely. Mimicking such a catalytically active pocket with artificial, non-peptidic catalysts represents a major challenge.
- We achieved a four step synthesis of the sesquiterpene natural product presilphiperfolan-1b-ol in only four steps, with the key cyclization (see below) taking place inside the supramolecular capsule catalyst. This natural product was previously only available via a long synthetic sequence (13 linear steps).
J. Am. Chem. Soc. 2020, 142, 5894.
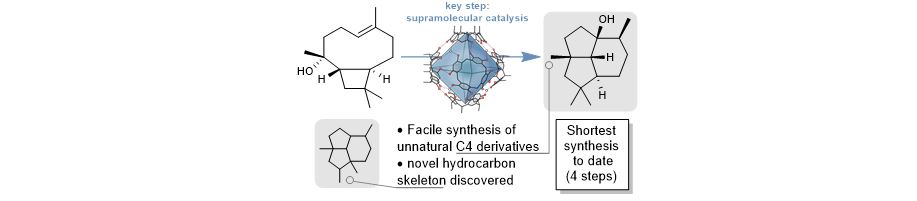
- Recently, we demonstrated that we can explore the large terpenoid chemical space that Nature is not able to access, by utilizing heavily modified substrates which are not tolerated by terpene cyclases.
Angew. Chem. Int. Ed. 2023, e202218625. - Recently, we also reported the first example of an enantioselective tail-to-head terpene cyclization inside a molecule flask:
Angew. Chem. Int. Ed.2022, 61, e202203384. - More examples of sesquiterpene cyclizations:
Nat. Catal. 2018, 1, 609.
- For background information, see our review article:
Nat. Prod. Rep. 2019, 36, 1619. - For more examples, see publications.
C-H Oxidation
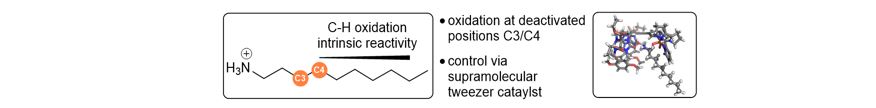
Challenge. The selective oxidation of un- or low-functionalized carbon frameworks, for example, those obtained by terpene cyclizations (see above), is still highly challenging. Currently, one main challenge in the field of C-H oxidation is directing the oxidation to unactivated/deactivated positions.
Vision. Oxidation of any desired position by directing the substrate, via substrate recognition, inside a molecular flask.
- Recently, we were able to override the intrinsic reactivity for C-H oxidation in alkyl ammonium substrates utilizing a supramolecular tweezer catalyst, favoring oxidation at the deactivated positions C3/C4.
Angew. Chem. Int. Ed. 2020, 59, 12387.
Chem. Eur. J. 2023, e202203480.
- Currently, we focus on the oxidation of unfunctionalized carbon frameworks.
Examples of further reactions catalyzed inside a molecular capsule
- Polyether cyclizations
Chem. Sci. 2022, 13, 10273. - Activation of benzylic and tertiary alkyl (sp3)C-F bonds
Front. Chem. 2019, 6, 639. - Carbonyl-Olefin metathesis
Angew. Chem. Int. Ed. 2018, 57, 14589. - Iminium catalysis
RSC Adv. 2021, 11, 24607-24612.
J. Am. Chem. Soc. 2017, 139, 17500.
Angew. Chem. Int. Ed. 2016, 55, 7698.
Novel Supramolecular Host Structures
Our group also aims at expanding the scope of supramolecular capsule catalysis. We modify existing host systems to alter and better understand their properties, and also develop novel systems in order to address current challenges in the field.
- Resorcin[4]arene based Systems
Chem. Eur. J. 2021, 27, 4447.
Org. Lett. 2020, 22, 5506. - Glycoluril‐Derived Molecular Tweezer
Org. Biomol. Chem. 2021, 19, 3628-3633.
Chem. Eur. J. 2019, 25, 12900.