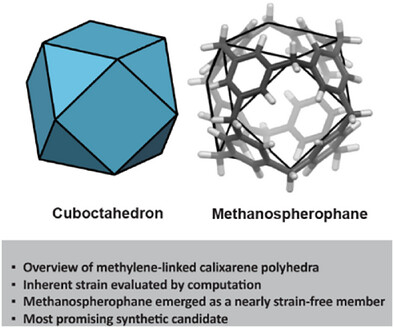
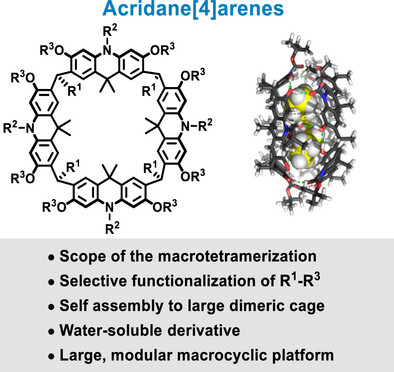
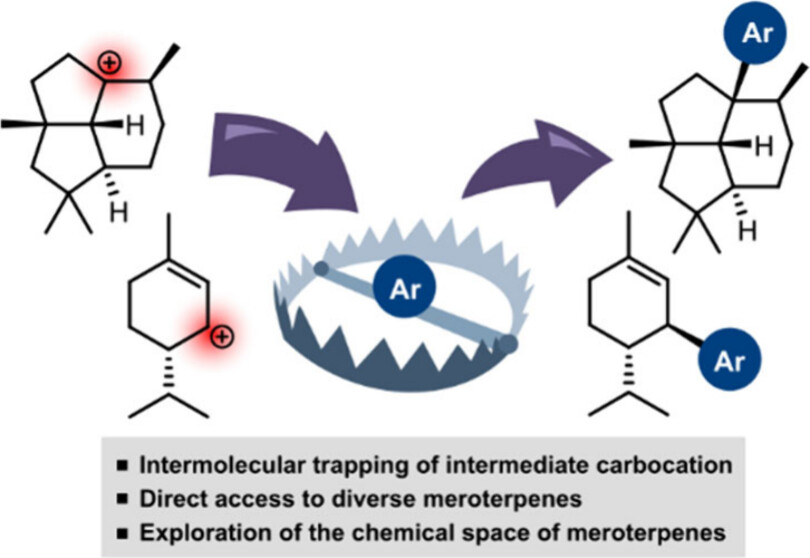
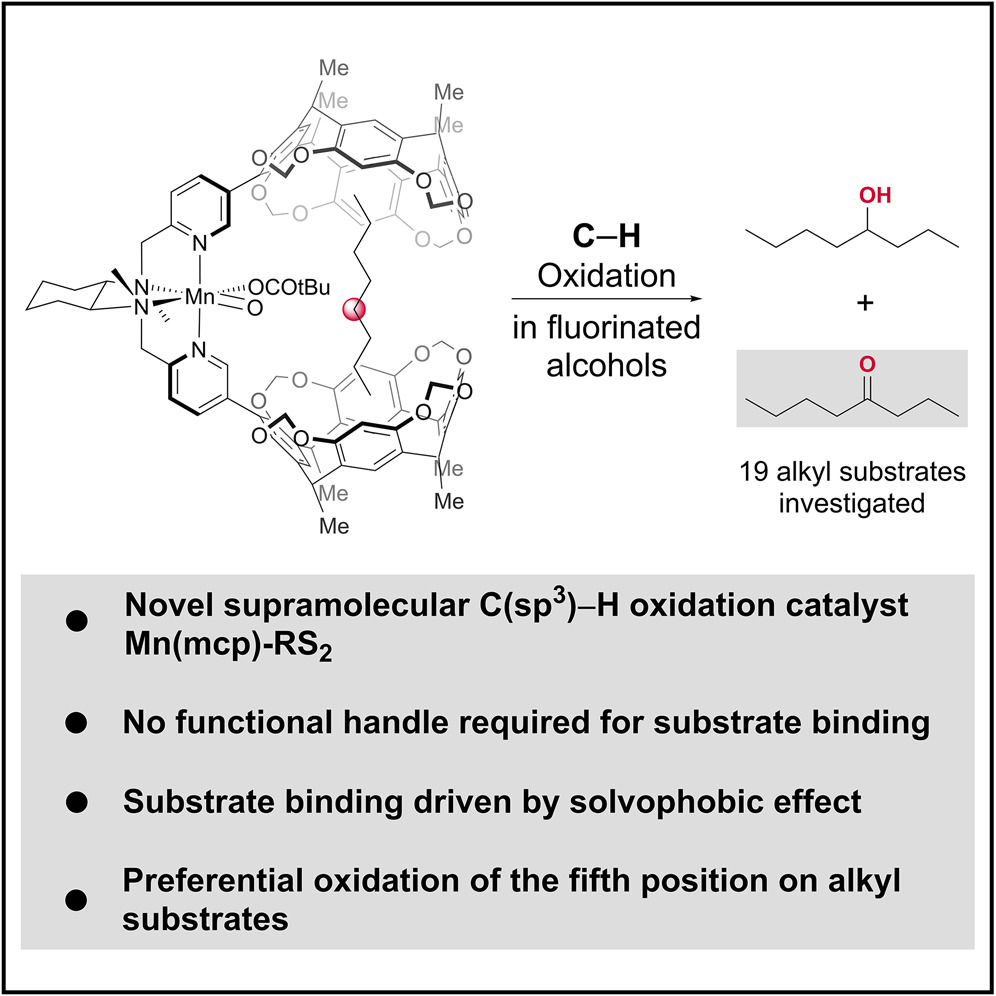
Publications
| 77. |
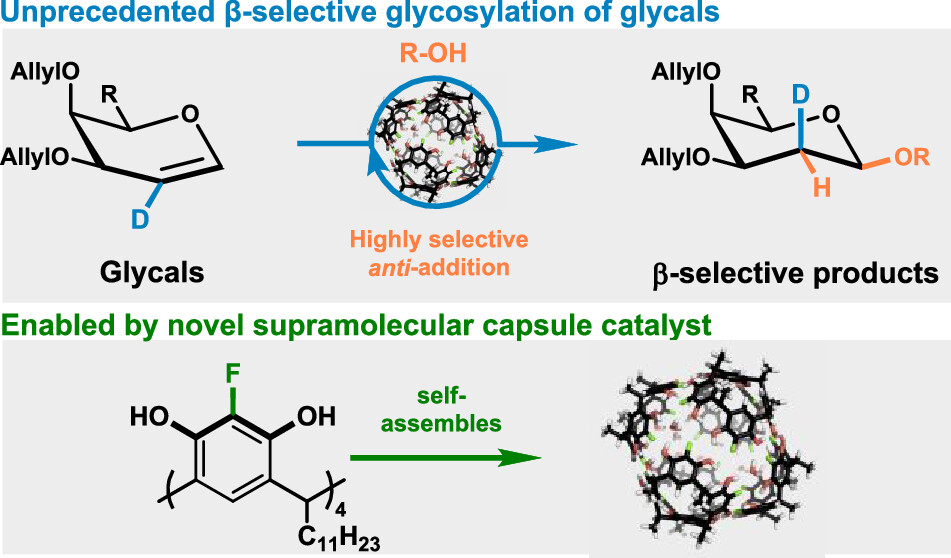
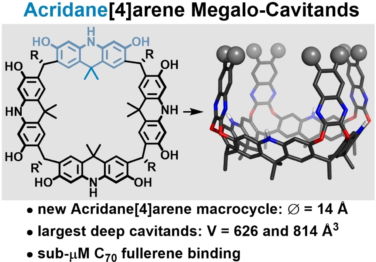
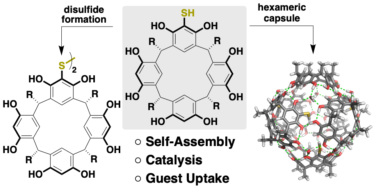
Kharchenko, A.; Cornu, I.; Goldfuss, B.; Tiefenbacher, K.* |
|
| 76. |
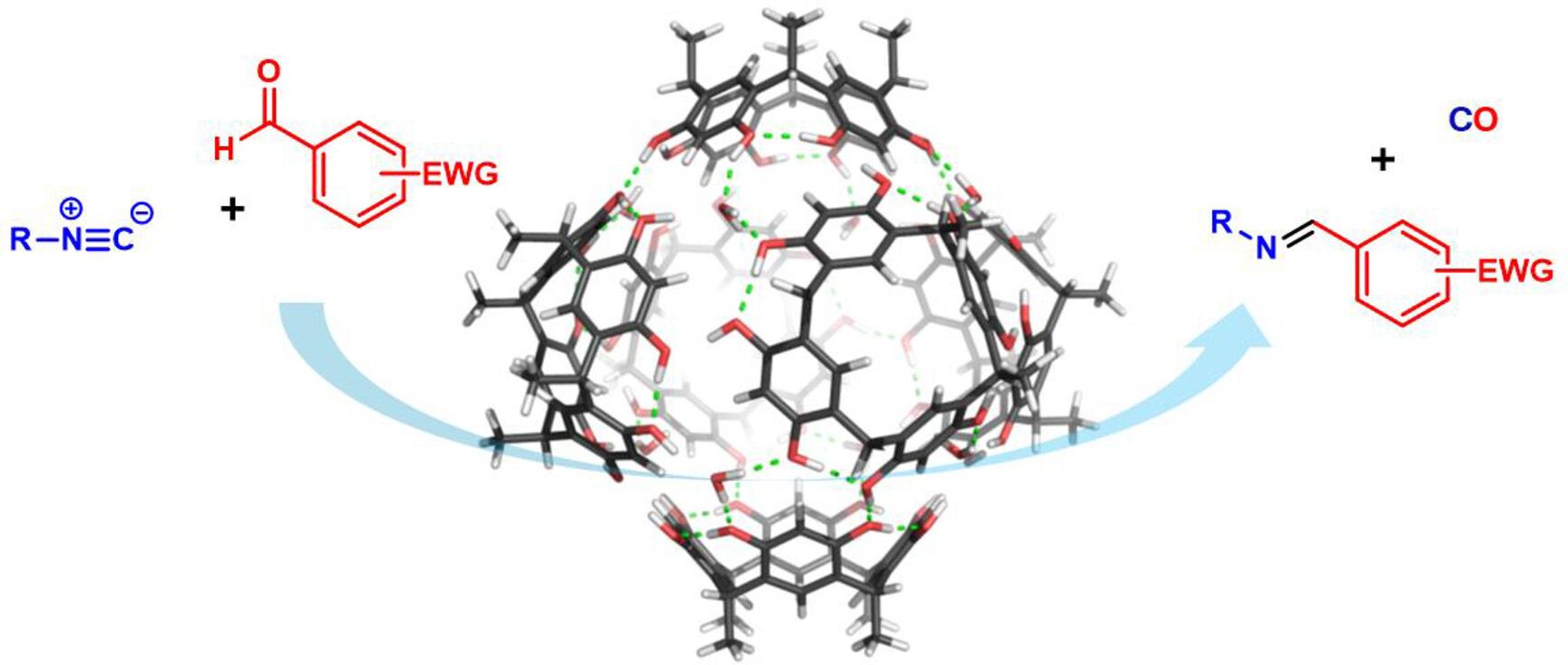
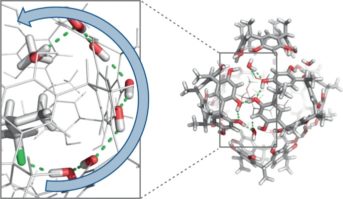
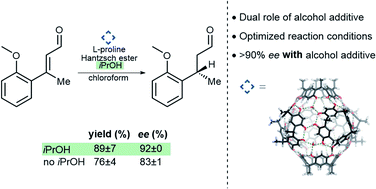
Höft, V.; Pfeuffer-Rooschüz, J.; López-Coll, R.; Prescimone, A.; Tiefenbacher, K.* |
|
| 75. |
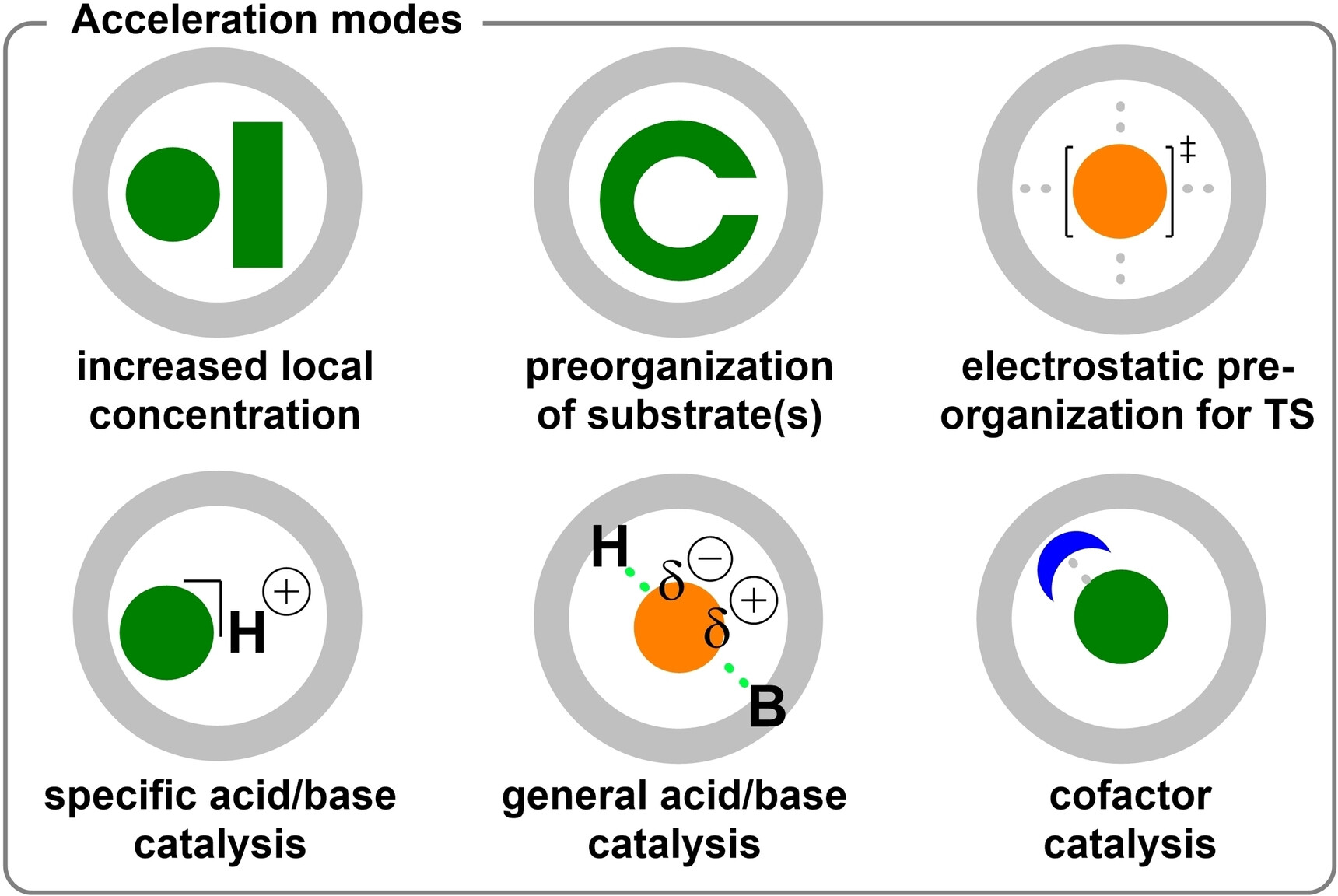
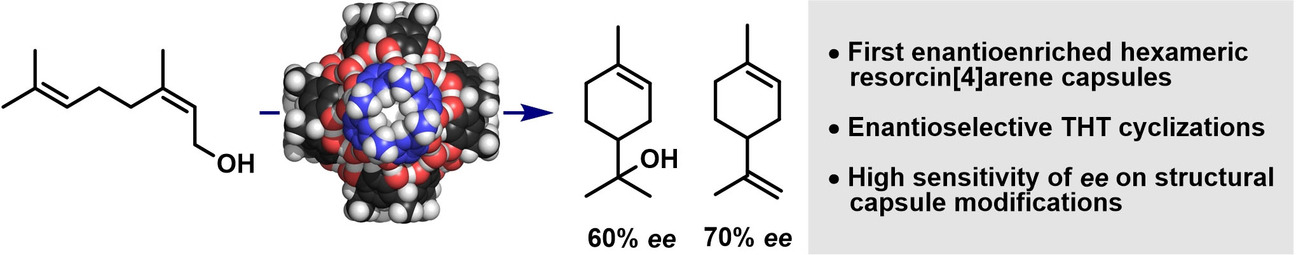
Cornu, I.; Häussinger, D.; Prescimone, A.; Tiefenbacher, K.* |
|
| 74. |
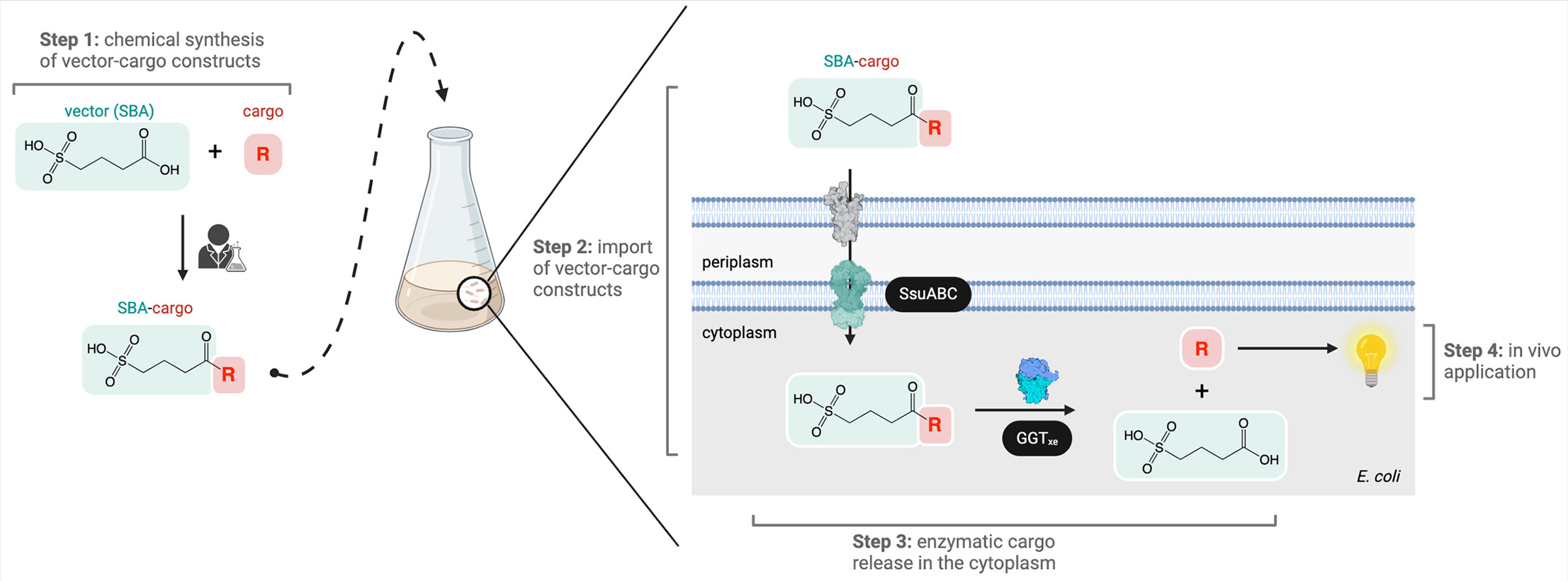
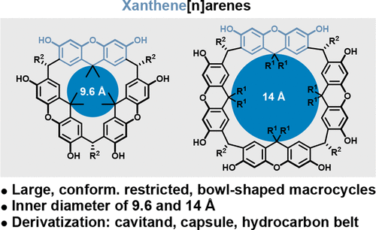
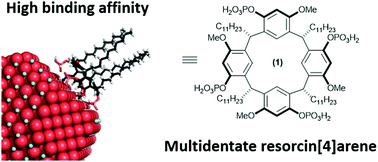
Lu, Y.; Knezevic, M.; Prescimone, A.; Goldfuss, B.; Tiefenbacher, K.* |
|
| 73. |
Li, T.-R.; Das, C.; Piccini, G. M.; Tiefenbacher, K. |
|
| 72. |
Fiorini, L.; Köster, J.; Piccini, G. M.; Goldfuss, B.; Prescimone, A.; Fabris, F.; Tiefenbacher, K.; Scarso, A. |
|
| 71. |
Syntrivanis, L-D.; Tiefenbacher, K. |
|
| 70. |
Rodríguez-Robles, E.; Müller, D.; Künzl, T.; Nemat, S. J.; Edelmann, M. P.; Srivastava, P.; Louis, D.; Groaz, E.; Tiefenbacher, K.; Roberts, T. M.; Herdewijn, P.; Marlière, P.; Panke, P. |
|
| 69. |
Persiani, G.; Sokolova, D.; Ivanov, R.; Merget, S.; Maintok, T.; Häussinger, D.; Tiefenbacher, K. |
|
| 68. |
Strassberger, A. F.; Zengaffinen, M. D.; Puigcerver, J.; Trapp, N.; Tiefenbacher, K. |
|
| 67. |
Li, T-R.; Das, C.; Cornu, I.; Prescimone, A.; Piccini, G.* and Tiefenbacher, K.* |
|
| 66. |
Muthwill, M. S.; Bina, M.; Paracini, N.; Coats, J. P.; Merget, S.; Yorulmaz Avsar, S.; Messmer, D.; Tiefenbacher, K.; Palivan, C. G. |
|
| 65. |
Cornu, I.; Syntrivanis, L-D.; Tiefenbacher, K.* |
|
| 64. |
Klucznik, T.; Syntrivanis, L.-D.; Baś, S.; Mikulak-Klucznik, B.; Moskal, M.; Szymkuć, S.; Mlynarski, J.; Gadina, L.; Beker, W.; Burke, M.; Tiefenbacher, K.; Grzybowski, B. |
|
| 63. |
Schmid, D.; Li, T-R.; Goldfuss, B.; Tiefenbacher, K.* |
|
| 62. |
Zenka, M.; Preinl, J.; Pertermann, E.; Lützen, A.; Tiefenbacher, K.* |
|
| 61. |
Li, T-R.; Piccini, G. M.; Tiefenbacher, K.* |
|
| 60. |
Némethová, I.; Schmid, D.; Tiefenbacher, K.* |
|
| 59. |
Knezevic, M.; Tiefenbacher, K.* |
|
| 58. |
Chen, H.; Li, T-R.; Sakai, N.; Besnard, C.; Guénée, L.; Pupier, M.; Viger-Gravel, J.; Tiefenbacher, K.; Matile, S.* |
|
| 57. |
Pfeuffer-Rooschüz, J.; Heim, S.; Prescimone, A.; Tiefenbacher, K.* |
|
| 56. |
Li, T-R.; Huck, F.; Piccini, G. M.; Tiefenbacher, K.* |
|
| 55. |
Sokolova, D.; Piccini, G.M.; Tiefenbacher, K.* |
|
| 54. |
Huck, F.; Catti, L.; Reber, G.L.; Tiefenbacher, K.* |
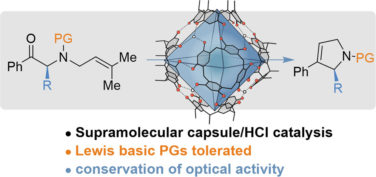 |
| 53. |
Pfeuffer-Rooschüz, J.; Schmid, L.; Prescimone, A.; Tiefenbacher, K.* |
|
| 52. |
Nemat, S. J.; Tiefenbacher, K.* |
|
| 51. |
Sokolova, D.; Tiefenbacher, K.* |
|
| 50. |
Hao, X.; Li, T-R.; Chen, H.; Gini, A.; Zhang, X.; Rosset, S.; Mazet, C.; Tiefenbacher, K.; Matile, S.* |
|
| 49. |
Nemat, S. J.; Van den Eynden, D.; Deblock, L.; Heilmann, M.; Köster, J. M.; Parvizian, M.; Tiefenbacher, K.*; De Roo, J.* |
|
| 48. |
Heilmann, M.; Knezevic, M.; Piccini, G. M.; Tiefenbacher, K.* |
|
| 47. |
Merget, S.; Catti, L.; Zev, S.; Major, D.T.; Trapp, N.; Tiefenbacher, K.* |
|
| 46. |
Némethová, I.; Syntrivanis, L.-D.; Tiefenbacher, K.* |
|
| 45. |
Nemat, S. J.; Jędrzejewska, H.; Prescimone, A.; Szumna, A.; Tiefenbacher, K.* |
|
| 44. |
Knezevic, M.; Heilmann, M.; Piccini, G. M.; Tiefenbacher, K.* |
|
| 43. |
Syntrivanis, L.-D.; Némethová, I.; Schmid, D.; Levi, S.; Prescimone, A.; Bissegger, F.; Major, D. T.; Tiefenbacher, K.* |
|
| 42. |
Bissegger, F. R.; Neuburger, M.; Tiefenbacher, K.* |
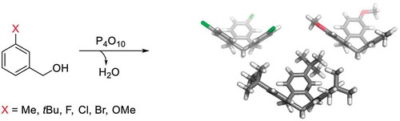 |
| 41. |
Merget, S.; Catti, L.; Piccini, G. M.; Tiefenbacher, K.* |
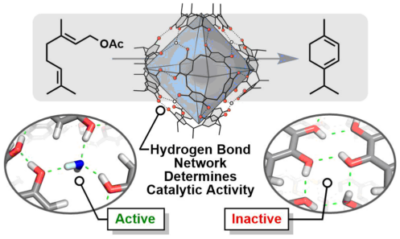 |
| 40. |
Heilmann, M.; Tiefenbacher, K.* |
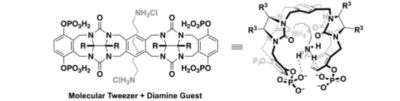 |
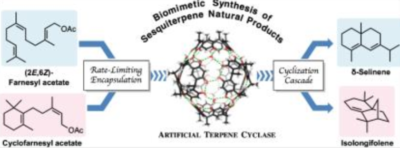
| 39. |
Zhang, Q.; Tiefenbacher, K.* |
 |
| 38. |
Zhang, Q.; Catti, L.; Syntrivanis, L.-D.; Tiefenbacher, K.* |
|
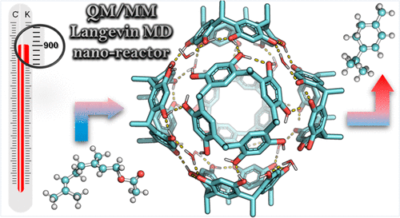
| 37. |
Pahima, E.; Zhang, Q.; Tiefenbacher, K.; Major, D. T. |
 |
| 36. |
Köster, J.; Häussinger, D.; Tiefenbacher, K.* |
|
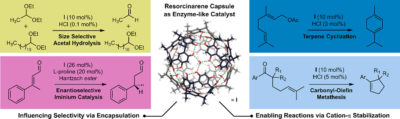
| 35. |
Zhang Q.; Catti, L.; Tiefenbacher, K.* |
 |
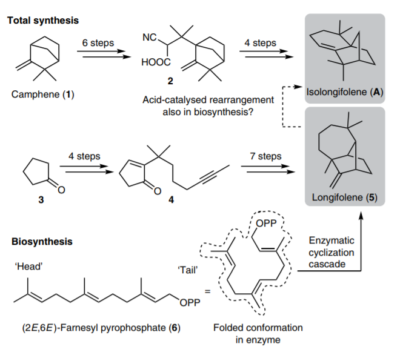
| 34. |
Zhang, Q.; Rinkel, J.; Goldfuss, B.; Dickschat, J.; Tiefenbacher, K.* |
 |
| 33. |
Thamm, I.; Tiefenbacher, K.; Rychlik, M. |
|
| 32. |
Köster, J.; Tiefenbacher, K.* |
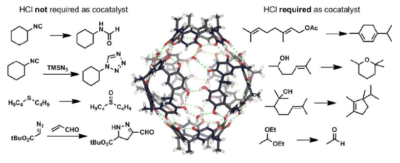 |
| 31. |
Catti, L.; Tiefenbacher, K.* |
|
| 30. |
Bräuer, T.; Zhang, Q.; Tiefenbacher, K.* |
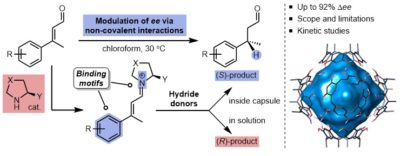 |
| 29. |
Lou, Y.; Steib, M.; Zhang, Q.; Tiefenbacher, K.; Horvath, A.; Jentys, A.; Liu, Y.; Lercher, J. A.*
J. Catal. 2017, 356, 147. |
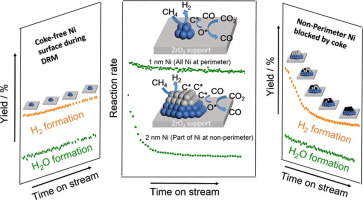 |
| 28. |
Zhang, Q.; Catti, L.; Pleiss, J.; Tiefenbacher, K.* |
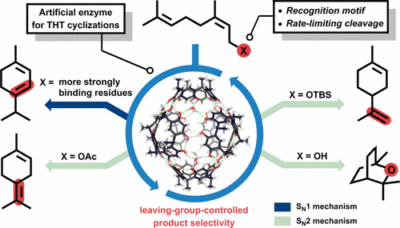 |
| 27. | Pollok, C. H.; Zhang, Q.; Tiefenbacher, K.; Merten, C. ChemPhysChem 2017, 18, 1987. “Chirality induction from a chiral guest to the hydrogen bonding network of its hexameric resorcinarene host capsule” |
|
| 26. |
Richers, J.; Pöthig, A.; Herdtweck, E.; Sippel, C.; Hausch, F.; Tiefenbacher, K.* |
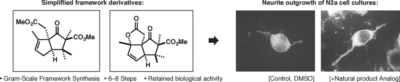 |
| 25. |
Catti, L.; Pöthig, A.; Tiefenbacher, K.* |
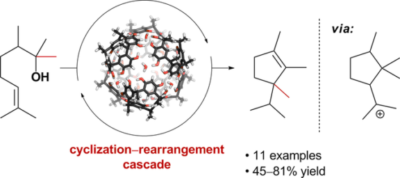 |
| 24. |
 |
|
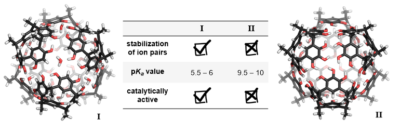
| 23. |
|
|
| 22. |
Catti L.; Bräuer T.; Zhang Q.; Tiefenbacher K.* |
|
| 21. |
Chem. Commun. 2016, 52, 11701.
“A six-step total synthesis of α-thujone and d6-α-thujone, enabling facile access to isotopically labelled metabolites” |
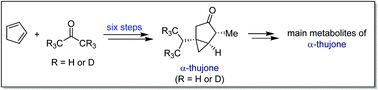 |
| 20. |
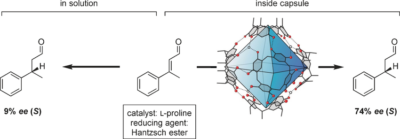
Bräuer, T.; Zhang, Q.; Tiefenbacher, K.*
Angew. Chem. Int. Ed. 2016, 55, 7698. “Iminium Catalysis inside a Self-Assembled Supramolecular Capsule: Modulation of Enantiomeric Excess” |
 |
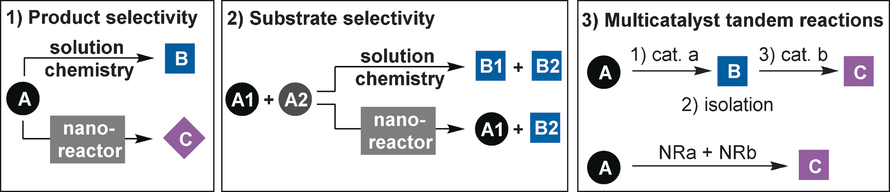
| 19. | Catti, L.; Zhang, Q.; Tiefenbacher, K.* Chem. Eur. J. 2016, 22, 9060. “Advantages of Catalysis in Self-Assembled Molecular Capsules” |
 |
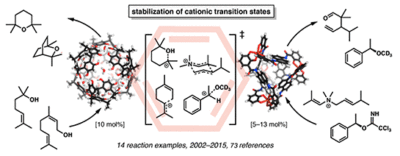
| 18. | Catti, L.; Zhang, Q.; Tiefenbacher, K.* Synthesis 2016, 48, 313. “Self-Assembled Supramolecular Structures as Catalysts for Reactions Involving Cationic Transition States” |
 |
| 17. | Zhang, Q.; Tiefenbacher, K.* Nature Chem. 2015, 7, 197. “Terpene cyclization catalysed inside a self-assembled cavity” (highlighted in “News and Views” Nature Chem. 2015, 7, 187.) |
 |
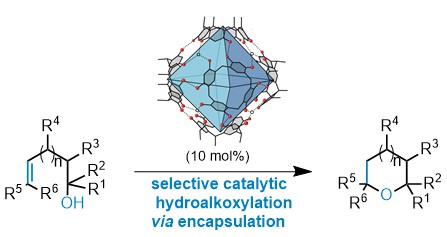
| 16. | Catti, L.; Tiefenbacher, K.* Chem. Commun. 2015, 51, 892. “Intramolecular hydroalkoxylation catalyzed inside a self-assembled cavity of an enzyme-like host structure” |
 |
| 15. | Räder, A..; Tiefenbacher, K.* Angew. Chem. Int. Ed. 2014, 53, 1206. (Highlight) “Tertiary Alcohols as Substrates for SN2-Like Stereoinversion” |
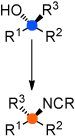 |
| 14. | Zhang, Q.; Tiefenbacher, K.* J. Am. Chem. Soc. 2013, 135, 16213. “Hexameric Resorcinarene Capsule is a Brønsted Acid: Investigation and Application to Synthesis and Catalysis” |
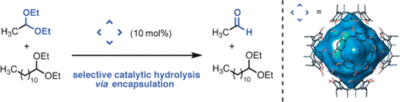 |
|
Postdoc &PhD work: |
||
| 13. | Tiefenbacher, K.; Zhang, K-d.; Ajami, D.; Rebek, J. J. Phys. Org. Chem. 2014, 28, 187. “Robust hydrogen-bonded capsules with stability in competitive media” |
|
| 12. | Wei, J.; Tiefenbacher, K.; Ajami, D.; Rebek, J. Chem. Sci. 2012, 3, 3022. “Complexes within complexes: hydrogen bonding in capsules” |
|
| 11. | Tiefenbacher, K.; Rebek, J. J. Am. Chem. Soc. 2012, 134, 2914. “Selective Stabilization of Self-Assembled Hydrogen-Bonded Molecular Capsules Through π-π Interactions” |
|
| 10. | Tiefenbacher, K.; Ajami, D.; Rebek, J. Angew. Chem. Int. Ed. 2011, 50, 12003. “Self-Assembled Capsules of Unprecedented Shapes” (highlighted in Science 2011, 333, 1361.) |
|
| 9. | Tiefenbacher, K.; Dube, H.; Ajami, D.; Rebek, J. Chem. Comm. 2011, 47, 7341. “A transparent photo-responsive organogel based on a glycoluril supergelator” |
|
| 8. | Tiefenbacher, K.; Trondlin, L.; Mulzer, J.; Pfaltz, A. Tetrahedron 2010, 66, 6508. “An expeditious asymmetric formal synthesis of the antibiotic platensimycin” |
|
| 7. | Tiefenbacher, K.; Gollner, A.; Mulzer, J. Chem. Eur. J. 2010, 16, 9616. “Syntheses and Antibacterial Properties of iso-Platencin, Cl-iso-Platencin and Cl-Platencin: Identification of a New Lead Structure” |
|
| 6. | Magauer, T.; Mulzer, J.; Tiefenbacher, K. Org. Lett. 2009, 11, 5306. “Total Syntheses of (+)-Echinopine A and B: Determination of Absolute Stereochemistry” |
|
| 5. | Tiefenbacher, K.; Mulzer, J. J. Org. Chem. 2009, 74, 2937. “A Nine-Step Total Synthesis of (–)-Platencin” |
|
| 4. | Tiefenbacher, K.; Mulzer, J. Angew. Chem. Int. Ed. 2008, 47, 6199. “Short Formal Synthesis of (–)-Platencin” |
|
| 3. | Tiefenbacher, K.; Mulzer, J. Angew. Chem. Int. Ed. 2008, 47, 2548. “Synthesis of Platensimycin (Minireview)” |
|
| 2. | Tiefenbacher, K.; Mulzer, J. Angew. Chem. Int. Ed. 2007, 46, 8074. “Protecting-Group-Free Formal Synthesis of Platensimycin” |
|
| 1. | Tiefenbacher, K.; Arion, V. A.; Mulzer, J. Angew. Chem. Int. Ed. 2007, 46, 2690. “A Diels-Alder Approach to (–)-Ovalicin” |
|